Epigenetic Regulation of the Tumour Suppressor RASSF1A in Bone Cancer Cells: DNA Methylation Study DNA methylation study
Main Article Content
Abstract
Objective: Osteosarcoma is a bone cancer that affects children and adolescents. The RASSF1A is a tumor suppressor capable of mediating the regulation of cell cycle arrest, migration, including apoptosis. It is the most continually silenced gene that contributes to human cancer. Furthermore, RASSF1A functions as a scaffold protein that can regulate microtubules network and bind apoptotic kinases MST1 and MST2 via the Sav-RASSF-Hippo domain. Epigenetic inactivation of genes by DNA methylation is a key factor regulating gene expression and genomic stability. Our aim was to study the RASSF1A gene promoter methylation in three osteosarcomas (U2OS, Saos-2, and MG-63), two Ewing Sarcoma (A-673 and SK-ES-1), and one-fibrosarcoma (HT-1080) cell lines.
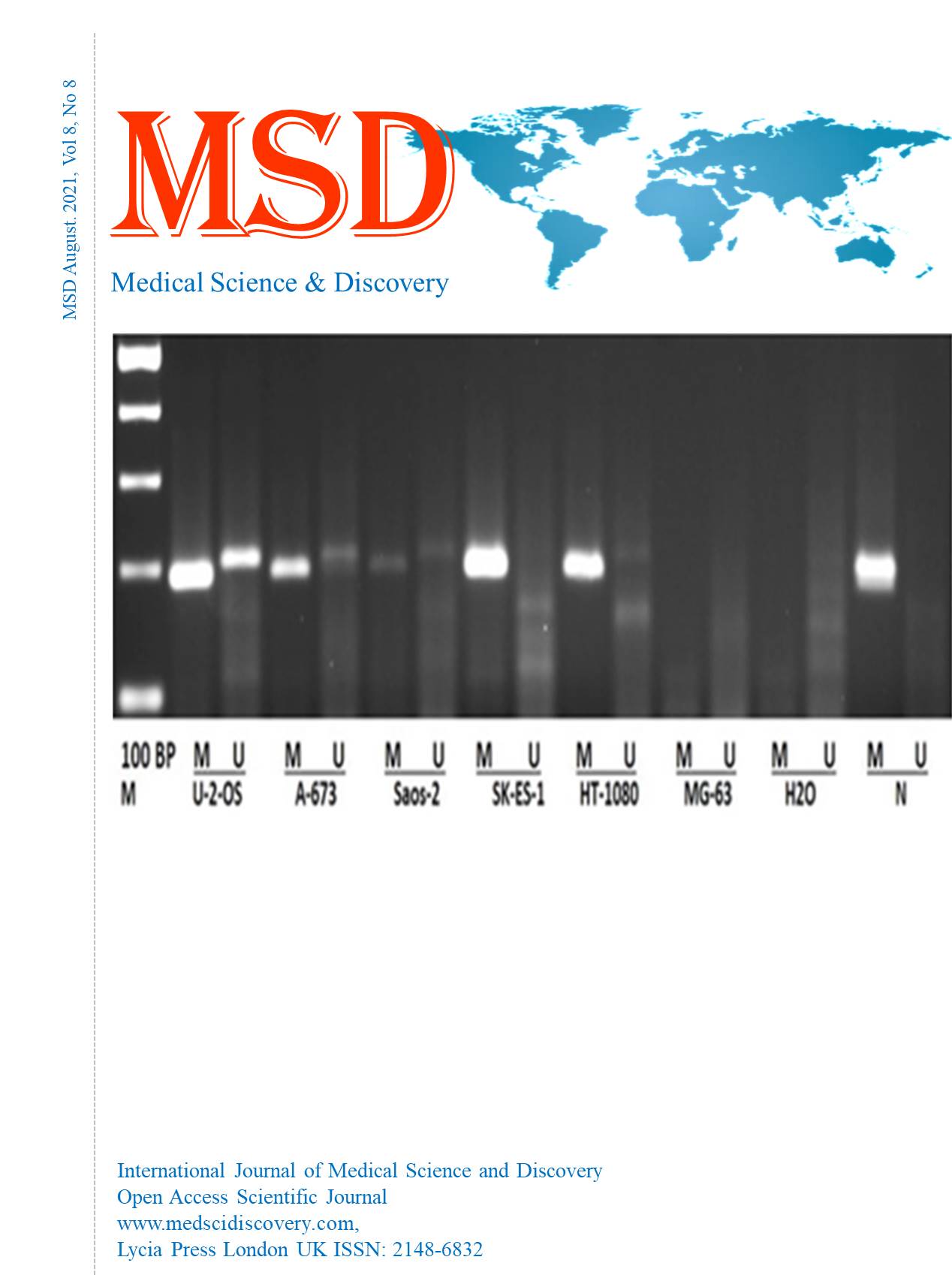
Materials and Methods: Three osteosarcomas (U2OS, Saos-2, and MG-63), two Ewing Sarcoma (A-673 and SK-ES-1), and one-fibrosarcoma (HT-1080) cell lines were used to study RASSF1A gene promoter methylation, using bisulphite conversion of DNA, followed by methylation-specific polymerase chain reaction (PCR)
Results: The RASSFIA’s gene promoter methylation was established as a frequent event. Hypermethylation of RASSF1A promoter, was detected in five out of six studied cell lines.
Conclusions: These results demonstrated that altering the Sav-RASSF1-Hippo may be accomplished through hypermethylation of RASSF1A and may play an essential role in Ewing’s sarcoma and Osteosarcoma. The methylation pattern of Sav-RASSF1-Hippo tumor suppressor pathway in human bone cancer along with RASSF1A expression with its effector proteins merits further investigation. This may reveal how the RASSFIA has a physiological signal transduction, including how the process of its deregulation can contribute to transformation of the cell, eventually leading to the incorporation of novel therapeutic options with improved prognosis for bone cancer.
Downloads
Article Details
Accepted 2021-07-16
Published 2021-08-02
References
Widhe B, Widhe T. Initial Symptoms and Clinical Features in Osteosarcoma and Ewing Sarcoma. The Journal of Bone & Joint Surgery. 2000; 82(5): 667-667.
Cho WH, Song WS, Jeon DG, Kong CB, Kim MS, Lee JA, Yoo JY, Do Kim J, Lee SY. Differential presentations, clinical courses, and survivals of osteosarcomas of the proximal humerus over other extremity locations. Annals of surgical oncology.2010; 17(3): 702-708.
Fagioli F, Aglietta M, Tienghi A, Ferrari S, Del Prever AB , Vassallo E, Palmero A, Biasin E, Bacci G, Picci P, Madon E. High-dose chemotherapy in the treatment of relapsed : an Italian sarcoma group study. Journal of Clinical Oncology. 2002; 20(8): 2150-2156.
Overholtzer M, Rao PH, Favis R, Lu XY, Elowitz MB, Barany F, Ladanyi M, Gorlick R, Levine AJ. The presence of p53 mutations in human osteosarcomas correlates with high levels of genomic instability. Proceedings of the National Academy of Sciences. 2003;100(20): 11547-11552.
Selvarajah S,Yoshimoto M, Maire G, Paderova J, Bayani J, Squire JA, Zielenska M. Identification of cryptic microaberrations in osteosarcoma by high-definition oligonucleotide array comparative genomic hybridization. Cancer genetics and cytogenetics.2007; 179(1): 52-61.
Jones PA, Baylin SB. The epigenomics of cancer. Cell.2007;128(4): 683-692.
Yoo CB, Jones PA. Epigenetic therapy of cancer: past, present and future. Nature reviews Drug discovery. 2006; 5(1): 37-50.
Suzuki MM, Bird A. DNA methylation landscapes: provocative insights from epigenomics. Nature Reviews Genetics. 2008; 9(6): 465-476.
Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4): 693-705.
Szyf M. The role of DNA hypermethylation and demethylation in cancer and cancer therapy. Current Oncology. 2008; 15(2): 72.
Fuks F. DNA methylation and histone modifications: teaming up to silence genes. Current opinion in genetics & development. 2005; 15(5): 490-495.
Saxonov S, Berg P, Brutlag DL. A genome-wide analysis of CpG dinucleotides in the human genome distinguishes two distinct classes of promoters. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(5): 1412-1417.
Fatemi M, Pao MM, Jeong S, Gal-Yam EN, Egger G, Weisenberger DG, Jones PA. Footprinting of mammalian promoters: use of a CpG DNA methyltransferase revealing nucleosome positions at a single molecule level. Nucleic acids research.2005; 33(20): e176-e176.
Malzkorn B, Wolter M, Riemenschneider MJ, Reifenberger G. Unraveling the gliomaepigenome: from molecular mechanisms to novel biomarkers and therapeutic targets. Brain Pathol. 2011; 21(6): 619-632.
Hoffmann MJ, Schulz WA. Causes and consequences of DNA hypomethylation in human cancer. Biochemistry and cell biology.2005;83(3): 296-321.
Rodríguez-Paredes M, Esteller M. Cancer epigenetics reaches mainstream oncology. Nature medicine.2011; 17(3): 330-339.
Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nature Reviews Cancer. 2013;13(4): 246-257.
Pan D. The hippo signaling pathway in development and cancer. Developmental cell. 2010;19(4): 491-505.
Seidel C, Schagdarsurengin U, Blümke K, Würl P, Pfeifer GP, Hauptmann S, Taubert H, Dammann R. Frequent hypermethylation of MST1 and MST2 in soft tissue sarcoma. Molecular carcinogenesis.2007; 46(10): 865-871.
Morrissey C, Martinez A, Zatyka M, Agathanggelou A, Honorio S, Astuti D, Morgan NV, Moch H, Richards FM, Kishida T. Epigenetic inactivation of the RASSF1A 3p21. 3 tumor suppressor gene in both clear cell and papillary renal cell carcinoma. Cancer research. 2001;61(19): 7277-7281.
Hesson LB, Dunwell TL, Cooper WN, Catchpoole D,. Brini AT, Chiaramonte R, Griffiths M, Chalmers AD, Maher ER, Latif F. The novel RASSF6 and RASSF10 candidate tumour suppressor genes are frequently epigenetically inactivated in childhood leukaemias. Mol Cancer. 2009;8(1): 42.
Steinmann K, Sandner A, Schagdarsurengin U, Dammann RH. Frequent promoter hypermethylation of tumor-related genes in head and neck squamous cell carcinoma. Oncology reports. 2009; 22(6): 1519-1526.
Schagdarsurengin U, Richter AM, Hornung J, Lange C, Steinmann K, Dammann RH. Frequent epigenetic inactivation of RASSF2 in thyroid cancer and functional consequences. Mol Cancer. 2010;9(1): 264.
Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. New England Journal ofMedicine. 2003;349(21): 2042-2054.
Korbie D J, Mattick JS. Touchdown PCR for increased specificity and sensitivity in PCR amplification. Nature Protocols. 2008;3(9): 1452-1456.
Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nature reviews genetics. 2002;3(6): 415-428.
Li, Y., Q. Wei, F. Cao and X. Cao. Expression and promoter methylation of the RASSF1A gene in sporadic breast cancers in Chinese women. Oncology reports. 2008;19(5): 1149-1153.
Seley G, Anna TB, Sumathi V, Abdullah A, Ashraf D, Elena A, Takeshi K, Toru H, Smadar A, Robert G, Eamonn RM, Farida L . RASSF2 methylation is a strong prognostic marker in younger age patients with Ewing sarcoma. Epigenetics. 2013; 8(9): 893–898.
Lim S, Yang MH, Park JH, Nojima T, Hashimoto H, Unni KK, Park Y-K. Inactivation of the RASSF1A in osteosarcoma. Oncology reports. 2003;10(4): 897-901.
Malpeli G, Innamorati G, Decimo I, Bencivenga M, Nwabo Kamdje AH, Perris R, Bassi C, Methylation Dynamics of RASSF1A and Its Impact on Cancer. Cancers. 2019;11:959.
Dubois F, Bergot E, Levallet G. Cancer and RASSF1A/RASSF1C, the Two Faces of Janus. Trends Cancer. 2019; 5: 662–665.
Rajabi H, Hata T, Li W, Long MD, Hu Q, Liu S, Raina D, Kui L, Yazumizu Y, Hong D. MUC1-C represses the RASSF1A tumor suppressor in human carcinoma cells. Oncogene. 2019; 38(47):7266-7277.
Donninger H, Schmidt ML, Mezzanotte J, Barnoud T, Clark GJ. Ras signaling through RASSF proteins. Semin. Cell Dev. Biol. 2016; 58: 86–95.
Fallahi, E, O’Driscoll NA, Matallanas D. The MST/Hippo Pathway and Cell Death: A Non-Canonical Affair. Genes. 2016; 7: 28.
Donninger H, Vos MD, Clark GJ. The RASSF1A tumor suppressor. J. Cell Sci. 2007; 120: 3163–3172.
Naofumi A, Hideyuki T, Satoshi Y, Hironori T, Naoko H, Takashi K, Akihiko Y, Eisuke K, Robert N, Morio M, Masaya N, Hitoshi I, Akira K, Tadashi K, Toshikazu U. Epigenetic reprogramming underlies efficacy of DNA demethylation therapy in osteosarcomas. Scientific Reports. 2019; 9(1):20360.
Dammann RH, Richter AM, Jimenez AP, Woods M, Kuster M, Witharana C. Impact of Natural Compounds on DNA Methylation Levels of the Tumor Suppressor Gene RASSF1A in Cancer. Int. J. Mol. Sci.2017; 18: 2160.
Harada K, Toyooka S, Maitra A, Maruyama R, Toyooka KO, Timmons CF, Tomlinson GE, Mastrangelo D, Hay RJ, Minna JD, Gazdar AF. Aberrant promoter methylation and silencing of the RASSF1A gene in pediatric tumors and cell lines. Oncogene. 2002;21(27): 4345-4349.
Dammann R, Schagdarsurengin U, Liu L, Otto N, Gimm O, Dralle H, Boehm PO, Pfeifer GP, Hoang-Vu C. Frequent RASSF1A promoter hypermethylation and K-ras mutations in pancreatic carcinoma. Oncogene. 2003; 22(24): 3806-3812.
Aruna B, Srinivas S, Jessy M, Harshada M, Ruchi A. Rassf Proteins as Modulators of Mst1 Kinase Activity. Sci Rep. 2017; 7: 45020.
Lucía G-G, Stephanie M, Walter K, David M. RASSF1A Tumour Suppressor: Target the Network for Effective Cancer Therapy. Cancers. 2020; 12: 229.
Huang K, Huang S, Chen I, Liao C, Wang H, Hsieh L. Methylation of RASSF1A, RASSF2A, and HIN-1 is associated with poor outcome after radiotherapy, but not surgery, in oral squamous cell carcinoma. Clin Cancer. 2009;15:4174–4180.
Ohshima J, Haruta M, Fujiwara Y, Watanabe N, Arai Y, Ariga T, Okita H, Koshinaga T, Oue T, Hinotsu S, Nakadate H, Horie H, Fukuzawa M, Kaneko Y. Methylation of the RASSF1A promoter is predictive of poor outcome among patients with Wilms tumor. Pediatr Blood Cancer. 2012;59:499–505.
Zhang CY, Zhao YX, Xia RH, Han J, Wang BS, Tian Z, Wang LZ, Hu YH, Li J. RASSF1A promoter hypermethylation is a strong biomarker of poor survival in patients with salivary adenoid cystic carcinoma in a Chinese population. PLoS One. 2014;9:e110159.