Bacterial contamination of propofol vials: The second report from Turkey
Main Article Content
Abstract
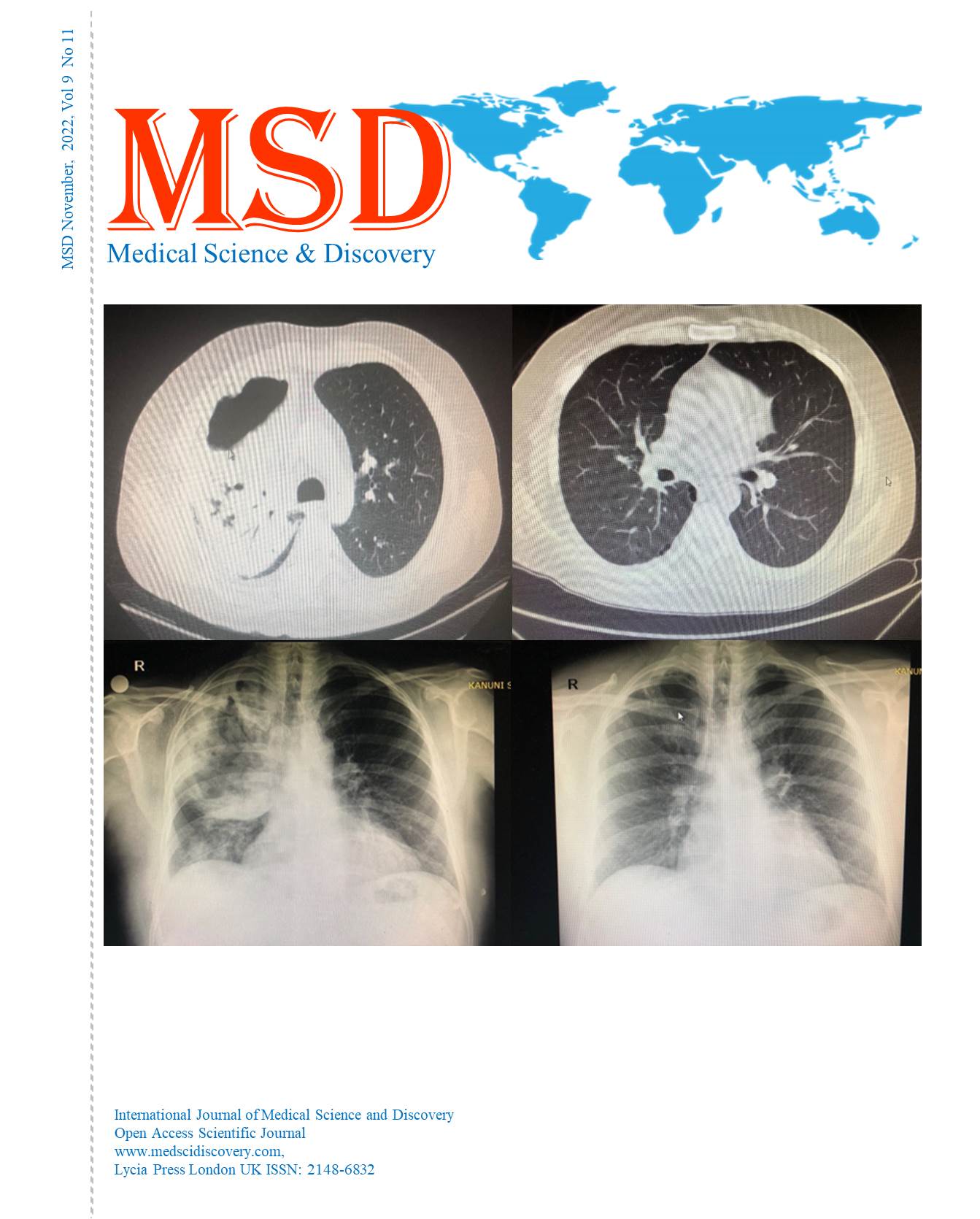
Objective: In this study, we look at the case report of an outbreak of sepsis in patients who underwent upper gastrointestinal endoscopy or colonoscopy during three consecutive days. Twelve patients had diagnostic procedures in the endoscopy unit between 05 May 2018 and 07 May 2018. Of the 12 patients, three had upper gastrointestinal endoscopy, six had a colonoscopy, and three had a combination of two procedures. Within two days of discharge, five patients diagnosed with SIRS and referred with fever as the major sign were hospitalized to the infectious diseases clinic. In our Endoscopy Unit, media, drug, and material cultures were taken for microbiological analysis, and microbial agents were searched. Growth was detected only in the propofol drawn into the syringe that was used on the patient. This study highlights the importance of strict compliance with aseptic injection guidance and constant analysis of microbiological data
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Accepted 2022-11-24
Published 2022-11-28
References
Arduino MJ, Bland LA, McAllister SK, Aguero SM, Villarino ME, McNeil MM, et al. Microbial growth and endotoxin production in the intravenous anesthetic propofol. Infect Control Hosp Epidemiol1991;12:535-9.PMID: 1940276.
Centers for Disease Control and Prevention. Postsurgical infections associated with an extrinsically contaminated intravenous anesthetic agent e California, Illinois, Maine, and Michigan, 1990. MMWR Morb Mortal Wkly Rep 1990;39:426-7.PMID: 2113169.
Bennett SN, McNeil MM, Bland LA, Arduino MJ, Villarino ME, Perrotta DM, et al. Postoperative infections traced to contamination of an intravenous anesthetic, propofol. N Engl J Med 1995;333:147-54. PMID: 7791816.
Cilli F, Nazli-Zeka A, Arda B, Sipahi OR, Aksit-Barik S, Kepeli N, et al. Serratia marcescens sepsis outbreak caused by contaminated propofol. Am J Infect Control. 2019;47(5):582-4. PMID: 30527282.
Henry B, Plante-Jenkins C, Ostrowska K. An outbreak of Serratia marcescens associated with the anesthetic agent propofol. AmJ Infect Control 2001;29:312-5.PMID: 11584257.
Tallis GF, Ryan GM, Lambert SB, Bowden DS, McCaw R, Birch CJ, et al. Evidence of patient-to-patient transmission of hepatitis C virus through contaminated intravenous anaesthetic ampoules. J Viral Hepat2003;10:234-9. PMID: 12753344.
Muller AE, Huisman I, Roos PJ, Rietveld AP, Klein J, Harbers JB, et al. outbreak of severe sepsis due to contaminated propofol: lessons to learn. J Hosp Infect 2010;76:225-30.
American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992;20:864-74.PMID: 20692067.
Zorrilla-Vaca A, Arevalo JJ, Escand on-Vargas K, Soltanifar D, Mirski MA. Infectious disease risk associated with contaminated propofol anesthesia, 1989-2014(1). Emerg Infect Dis 2016;22:981-92.PMID: 27192163; PMCID: PMC4880094.
Ersoz G, Uguz M, Aslan G, Horasan ES, Kaya A. Outbreak of meningitis due to Serratia marcescens after spinal anaesthesia. J Hosp Infect 2014;87:122-5.PMID: 24814159.
Us E, Kutlu HH, Tekeli A, Ocal D, Cirpan S, Memikoglu KO. Wound and soft tissue infections of Serratia marcescens in patients receiving wound care: a health care associated outbreak. Am J Infect Control 2017;45:443-7.PMID: 28063729.
Liu D, Zhang LP, Huang SF, Wang Z, Chen P, Wang H, et al. Outbreak of Serratia marcescens infection due to contamination of multiple-dose vial of heparinsaline solution used to flush deep venous catheters or peripheral trocars. J Hosp Infect 2011;77:175-6.PMID: 21194795.
Chemaly RF, Rathod DB, Sikka MK, Hayden MK, Hutchins M, Horn T, et al. Serratia marcescens bacteremia because of contaminated prefilled heparin and saline syringes: a multi-state report. Am J Infect Control 2011;39:521-4.PMID: 21492963.
Arslan U, Erayman I, Kirdar S, Yuksekkaya S, Cimen O, Tuncer I, et al. Serratia marcescens sepsis outbreak in a neonatal intensive care unit. Pediatr Int 2010;52:208-12.PMID: 19664012.
de Boer MG, Brunsveld-Reinders AH, Salomons EM, Dijkshoorn L, Bernards AT, van den Berg PC, et al. Multifactorial origin of high incidence of Serratia marcescens in a cardiothoracic ICU: analysis of risk factors and epidemiological characteristics. J Infect 2008;56:446-53. PMID: 18511122.